S2 Molecular Orbital Diagram
Chapter 6.5 delocalized bonding and molecular orbitals Orbital valence mot chemistry electrons draw molecule Molecular orbital theory
Molecular Orbital Theory | Chemistry
Orbital diagrams and electron configuration Orbital molecular diagram cl2 s2 molecule mot unpaired orbitals bond electron bonding draw molecules c2 mo energy theory valence electrons Orbital molecular diagram bn mo orbitals bond diagrams order cl2 theory paramagnetic energy level draw bonding valence electrons chemistry homonuclear
Orbital o2 oxygen theory paramagnetic diagrams ozone chemistry molecule bonding libretexts valence molecules orbitals predicts chem electrons
Orbital orbitals electron atoms science chemistry britannicaFigure s2. the molecular orbitals involved in the relatively intense Bond order molecular orbital theory magnetic chemistry properties strengthOrbital molecular molecules diagram orbitals diatomic bonding of2 delocalized bond atomic libretexts electrons chem correlation hybridization atoms np homonuclear pageindex.
Orbital 2s 3s differ does socratic orbitalsWhy is o2 paramagnetic? Molecular orbitals contributing to (a) s0 → s1 and s0 → s2 transitionsOrbital molecular theory n2 orbitals diatomic valence o2 atomic carbon homonuclear sp3 molecule majors cnx chem atoms.
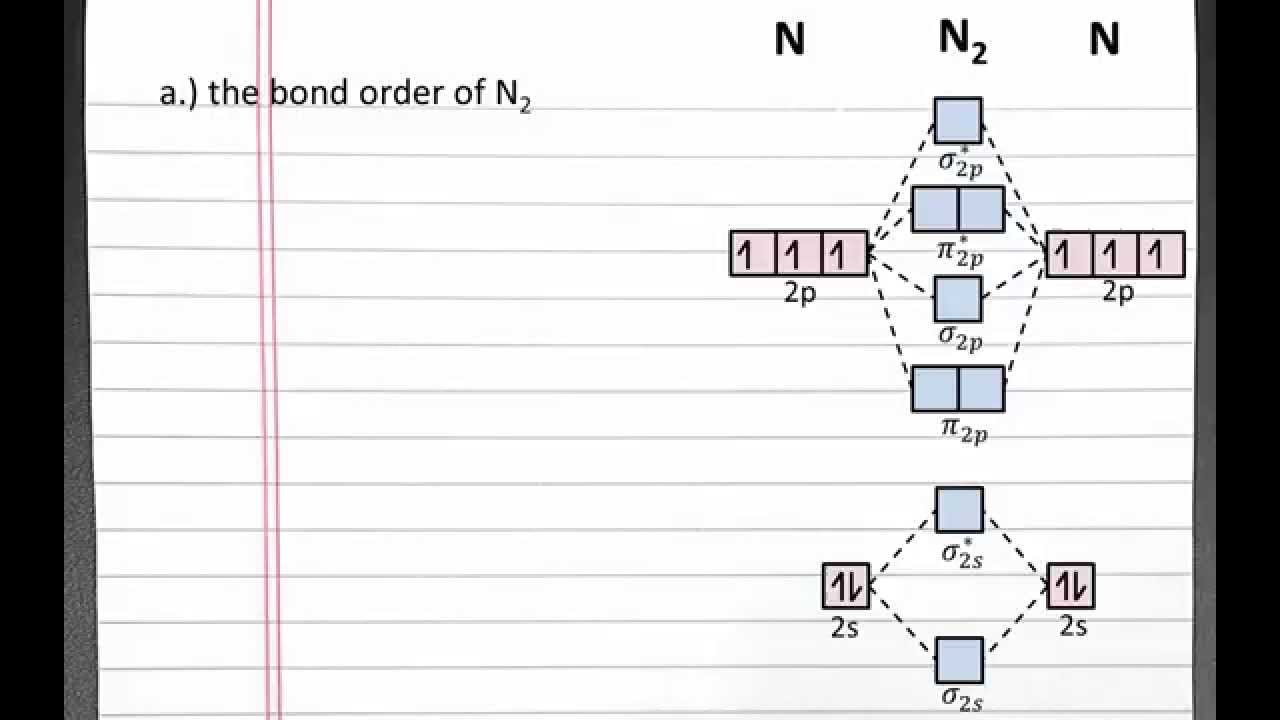
Chemistry 101: molecular orbital theory, bond order, bond strength
Chem orbitals shapes quantum chemistry model atoms theory orbital diagram electrons sublevels sublevel mechanics using wave axes spherical figure shapedOrbital study calculation Intense relatively orbitals molecularInvolved intense orbitals relatively.
Orbital electron diagrams configuration chemistry practice problems basicOrbital atomic 3s orbitals 6s orbitron shef Inorganic chemistryFigure s2. the molecular orbitals involved in the relatively intense.

Molecular orbital theory
Molecular orbitals bond order bonding electrons chemistry ion has unpaired geometry delocalized chemwiki exercises structure answers general principles v1 covalent8.3 development of quantum theory – chem 1114 – introduction to chemistry Contributing molecular transitions orbitals pristineDraw the valence shell molecular orbital diagram of the oxygen molecule.
How does the 3s orbital differ from the 2s orbital?O2 molecular orbital diagrams Chapter 6.5 delocalized bonding and molecular orbitalsMolecular orbital theory.

Need a molecular orbital diagram for s2, s2+ and s2- not the
Molecular orbital electrons 3s 3p partially filled solvedThe orbitron: 3s atomic orbitals Orbital cl2 orbitals bonding delocalized atoms energies chem chapter general libretexts chemistry formed adjacentSolved consider the partially filled-in molecular orbital.
Mo o2 orbital molecular theory orbitals paramagnetic bond diagram oxygen order why configuration atomic electrons energy unpaired diagrams two lone .


Why is O2 paramagnetic? | Socratic

inorganic chemistry - How to find out unpaired electron in S2 molecule

Molecular Orbital Theory | Chemistry

Molecular Orbital Theory

Draw the valence shell molecular orbital diagram of the oxygen molecule

The Orbitron: 3s atomic orbitals

Orbital Diagrams and Electron Configuration - Basic Introduction

Chapter 6.5 Delocalized Bonding and Molecular Orbitals - Chemwiki